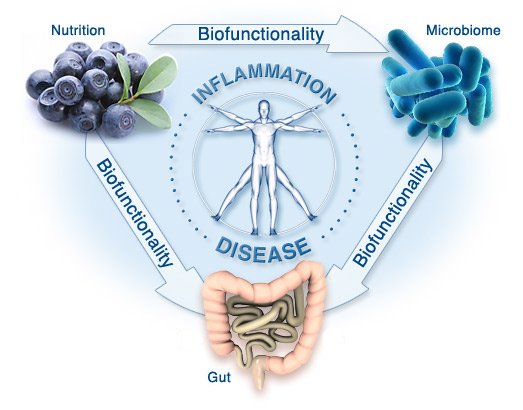
Scientific research continues to emphasize the importance intestinal microbiome plays in the health and immunity of the host organism. The food we consume is metabolized by the gut microbiota into small molecules that we absorb.
Over the past decade, a dysbiosis in the composition of gut microbiota has been found in patients suffering from inflammatory bowel disease (IBD). Specifically, faecalibacterium prausnitzii, a bacteria strain which is prevalent in healthy subjects has been found to be decreased in IBD patients. This bacteria has anti-inflammatory effects on the host, which points to the role of inflammation in IBD.
Fermentable fiber from diet is digested and fermented by the gut microbiota to make short-chain fatty acids. Among other effects, short-chain fatty acids induce anti-inflammatory T-regulatory cells. It is hypothesized that if one could increase the population of these cells by delivering more short-chain fatty acids, one might thus decrease inflammation.
Inflammation in the gut is also responsible for a leaky gut barrier, which means that the protective barrier in the intestinal lumen is damaged. This enables bacterial and microbiome products to permeate it and enter the abdominal cavity, reaching the liver through the portal vein. This leads to inflammatory changes in the liver and causes progression of disease.
Extending our understanding of treatments by fecal transplants to disease processes and reversing dysbiosis is the goal of future research.
(Source: https://www.md-fm.com/Vreport-vid-84-comefromback.html)
